Definition of Water (H₂O)
Water (H2O) is one of the most essential chemical substances for life and the environment. Water is a chemical substance composed of two hydrogen atoms and one oxygen atom, represented by the molecular formula H₂O. It is a colorless, odorless, and tasteless liquid under normal conditions and is essential for all known forms of life. Although chemically simple, water shows unique physical and chemical properties that make it vital for biological, chemical, and environmental processes.
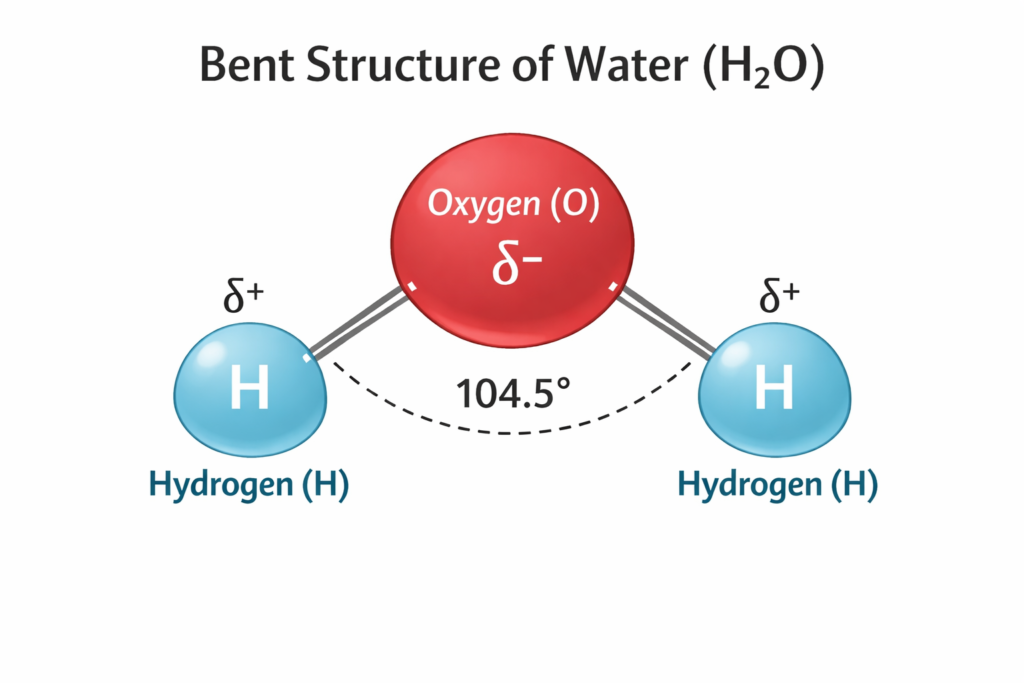
The molecular structure of water consists of one oxygen atom covalently bonded to two hydrogen atoms in a bent shape with a bond angle of about 104.5°. Oxygen is more electronegative than hydrogen, resulting in partial negative and positive charges within the molecule. This polarity makes water a polar compound and enables the formation of hydrogen bonds between neighboring water molecules.

Hydrogen bonding is responsible for many important properties of water, such as high boiling point, high specific heat, surface tension, cohesion, and adhesion. These properties help maintain stable temperatures in living organisms and natural environments. Because of its polarity, water can dissolve many ionic and polar substances and is therefore known as the universal solvent.
Biologically, water acts as a medium for metabolic reactions, transports nutrients, gases, and waste materials, and regulates body temperature. It is essential for processes like digestion, respiration, photosynthesis, and excretion. Environmentally, water plays a key role in the water cycle, supports aquatic and terrestrial ecosystems, and influences weather and climate.
In conclusion, water is a fundamental molecule that supports life, maintains environmental balance, and enables countless chemical and biological processes. Its structure, properties, and functions make it indispensable for all living organisms and the Earth as a whole.
Overview of Water (H₂O) and Its Importance
Water (H₂O) has a bent molecular structure with a bond angle of 104.5°. is one of the most important substances on Earth. It supports life, regulates climate, shapes landforms, and plays a central role in biological, chemical, and physical processes. From cellular metabolism to global weather systems, water is involved at every level. Understanding water is fundamental for students of biology, chemistry, environmental science, geography, and health sciences. Because of its unique physical and chemical properties, water (h2o) is often called the “universal solvent” and the “basis of life.”
Location and Occurrence of Water (H₂O)
Water (h2o) occurs naturally in many forms and locations:
- On Earth’s surface as oceans, seas, rivers, lakes, and wetlands
- Underground as groundwater and aquifers
- In the atmosphere as water vapor, clouds, and precipitation
- In polar regions as ice and glaciers
- Inside living organisms, making up a large percentage of cells and tissues
Approximately 71% of Earth’s surface is covered by water (H₂O), though most of it is saline and found in oceans.
Structure of Water (H₂O)
The structure of water (H₂O) is simple at the molecular level but highly significant in its effects. A water molecule consists of:
- One oxygen atom covalently bonded to two hydrogen atoms
- A bent (V-shaped) geometry with an angle of about 104.5°
- Polar covalent bonds due to unequal sharing of electrons
This structure gives water its polarity, which is responsible for many of its unique properties such as hydrogen bonding, high boiling point, and solvent ability.
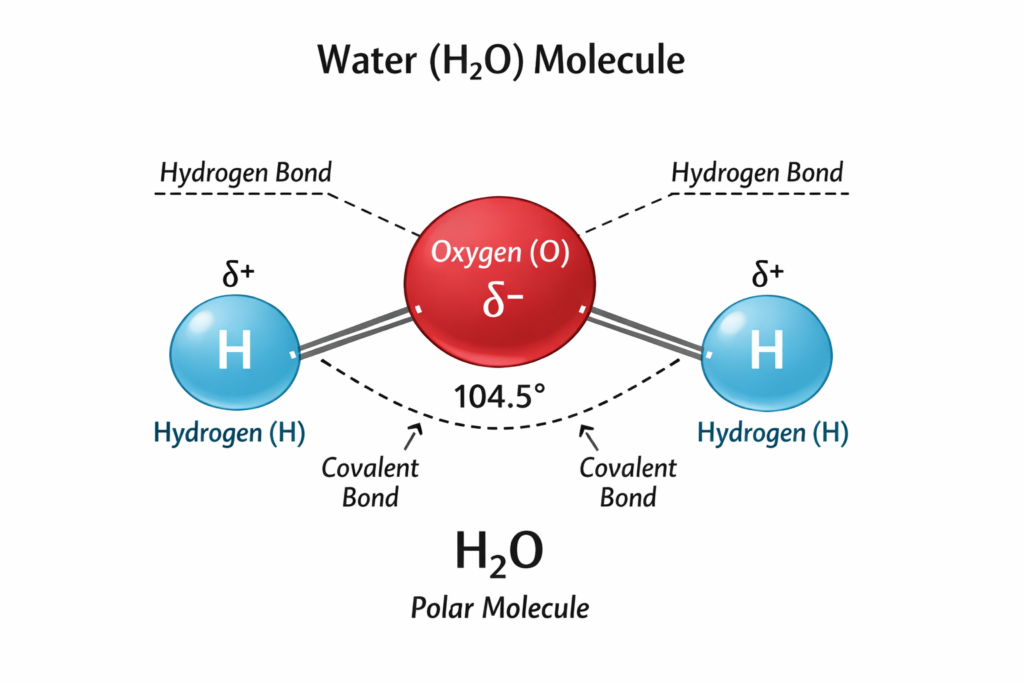
Diagram Explanation (Text Only)
In a typical diagram of a water molecule:
- The oxygen atom is shown at the center and is larger than hydrogen atoms
- Two hydrogen atoms are attached to the oxygen atom at an angle
- Partial negative charge (δ–) is indicated on oxygen
- Partial positive charges (δ+) are shown on hydrogen atoms
- Dotted lines between molecules represent hydrogen bonds
These labeled parts explain why water molecules attract each other and interact strongly with other polar substances.
Parts of Water (H₂O)
Hydrogen Atoms in Water (H₂O)
Definition:
Hydrogen atoms are fundamental components of many chemical compounds and play a crucial role in the structure and properties of water (H₂O). Hydrogen atoms are the two lightest atoms bonded to oxygen in a water molecule. Each hydrogen atom shares one electron with the oxygen atom, forming a covalent bond that contributes to the stability of the molecule. In water, hydrogen atoms carry a partial positive charge due to the higher electronegativity of oxygen, which allows them to participate in hydrogen bonding. This interaction is essential for many unique properties of water, including cohesion, surface tension, and its ability to support biological processes.
Location:
They are positioned on either side of the oxygen atom, forming covalent bonds.
Structure:
Each hydrogen atom shares one electron with oxygen, forming a single covalent bond.
Function:
Hydrogen atoms help form hydrogen bonds with nearby water molecules and other polar compounds.
Importance:
They are essential for water’s polarity, cohesion, and role in acid–base reactions.
Oxygen Atom in Water (H₂O)
Definition:
The oxygen atom is a key element in the structure and behavior of water and many biological molecules. Oxygen is a highly electronegative atom that forms the central part of the water molecule. In a water molecule, the oxygen atom is covalently bonded to two hydrogen atoms and possesses lone pairs of electrons, which give the molecule a bent shape. Because oxygen strongly attracts shared electrons, it develops a partial negative charge, making the water molecule polar. This property allows oxygen to form hydrogen bonds with nearby molecules, which is essential for water’s solvent ability, thermal stability, and its vital role in chemical and biological processes.
Location:
It lies at the center of the molecule, bonded to both hydrogen atoms.
Structure:
Oxygen has two lone pairs of electrons and forms two covalent bonds, creating a bent shape.
Function:
It attracts electrons more strongly, creating partial charges in the molecule.
Importance:
Oxygen is responsible for water’s polarity, solvent properties, and hydrogen bonding.
Hydrogen Bonds (Intermolecular Part) in Water (H₂O)
Definition:
Hydrogen bonds are important intermolecular forces that influence the physical and biological properties of water. Hydrogen bonds are weak attractions between the hydrogen of one water molecule and the oxygen of another. These bonds form because of the partial positive charge on hydrogen and the partial negative charge on oxygen. Although individually weak, hydrogen bonds collectively hold water molecules together, giving water its high boiling point, surface tension, cohesion, and thermal stability. They are essential for maintaining water in liquid form and for supporting life processes in living organisms.
Location:
They occur between neighboring water molecules.
Structure:
These are not true chemical bonds but electrostatic attractions.
Function:
They hold water molecules together in liquid form.
Importance:
Hydrogen bonding explains high boiling point, surface tension, and thermal stability of water.
Functions of Water (H₂O)
- Acts as a solvent for biochemical reactions
- Transports nutrients, gases, and waste materials
- Regulates body temperature through sweating and evaporation
- Participates directly in metabolic reactions such as hydrolysis
- Maintains cell shape and structure
- Supports photosynthesis in plants
- Helps regulate Earth’s climate
Importance and Applications of Water (H₂O)
Biological Importance:
Water makes up 60–90% of living cells. It is essential for digestion, circulation, excretion, and cellular reactions.
Chemical Importance:
Water is a universal solvent for ionic and polar compounds. It is involved in acid–base chemistry and redox reactions.
Environmental Importance:
Water drives the hydrological cycle, supports ecosystems, and influences weather and climate patterns.
Practical Importance:
Water is used in agriculture, industry, sanitation, energy production, and domestic activities such as cooking and cleaning.
Common Mistakes and Misconceptions About Water (H₂O)
- Thinking water is a simple substance without unique properties
- Believing all water on Earth is drinkable
- Confusing hydrogen bonds with covalent bonds
- Assuming water is non-reactive in chemical processes
- Ignoring the role of water in temperature regulation
Exam-Oriented Key Points on Water (H₂O)
- Chemical formula of water is H₂O
- Water molecule is polar and bent in shape
- Hydrogen bonding gives water unique properties
- Water has high specific heat and surface tension
- Essential for life and metabolic processes
Short Practice Questions
1. What is the molecular structure of water and why is it polar?
Answer: Water (H₂O) has the chemical formula H₂O with one oxygen atom covalently bonded to two hydrogen atoms in a bent shape (104.5°). Oxygen is more electronegative, creating partial charges (δ– on O, δ+ on H), so water is polar.
Diagram point: Water (H₂O) has a bent molecular structure with a bond angle of 104.5°, δ+ on H and δ– on O.
2. Explain the role of hydrogen bonding in water (H₂O).
Answer: Hydrogen bonding is the attraction between hydrogen of one water (H₂O) molecule and oxygen of another. It gives water high boiling point, surface tension, and cohesion, making water stable in liquid form.
Diagram point: Use dotted lines between H of one molecule and O of another.
3. List any four biological functions of water (H₂O).
Answer:
- Acts as a solvent for biochemical reactions
- Transports nutrients and wastes
- Regulates body temperature
- Essential for metabolic processes like photosynthesis
4. Why is water (H₂O) called the universal solvent?
Answer: Water (H₂O) is called the universal solvent because it dissolves most ionic and polar substances. Its polarity helps separate ions and molecules in solution.
Chemical point: Dissolves salts like NaCl into Na⁺ and Cl⁻ ions.
5. State two environmental roles of water (H₂O).
Answer:
- Maintains the water cycle (evaporation, condensation, rainfall)
- Supports ecosystems and climate regulation
Conclusion and Revision Summary of Water (H₂O)
Water (H₂O) is a fundamental substance that supports life, regulates natural systems, and enables countless chemical and biological processes. Due to its unique molecular structure, water shows properties that are essential for living organisms and the environment. A clear understanding of water (H₂O) helps students grasp key concepts in biology, chemistry, and environmental science and prepares them for both academic examinations and real-world applications.
You can also visit Earth Science
Frequently Asked Questions (FAQs)
1. Why is water (H₂O) essential for life?
Water (H₂O) provides a medium for biochemical reactions, transports substances, and maintains temperature balance in organisms.
2. What makes water (H₂O) a polar molecule?
The unequal sharing of electrons between oxygen and hydrogen creates partial charges, making water polar.
3. How does water (H₂O) regulate temperature?
Its high specific heat allows it to absorb and release heat slowly, stabilizing temperatures.
4. Is pure water (H₂O) found naturally?
Pure water (H₂O) is rare in nature; most natural water contains dissolved salts and gases.
5. Why is water (H₂O) important for the environment?
Water supports ecosystems, drives climate systems, and sustains plant and animal life.